-
In a first for Apple, FDA clears EKG device for Apple Watch
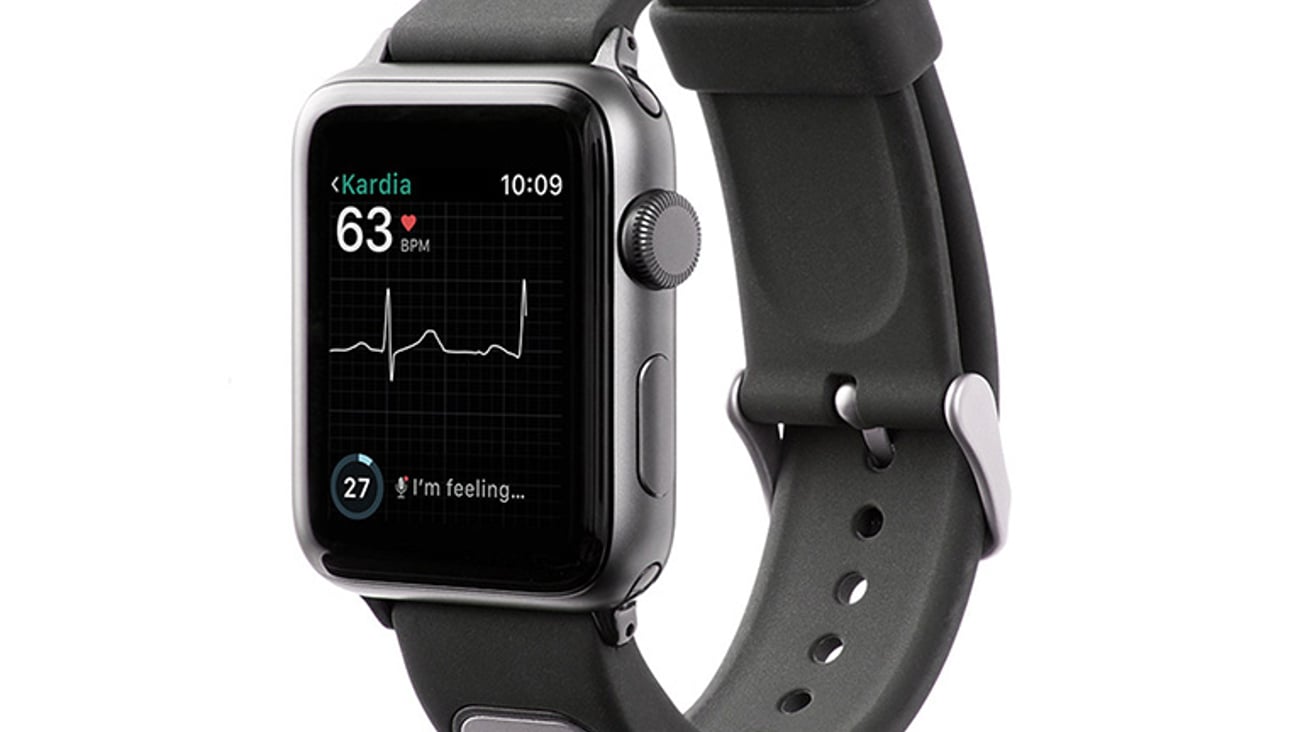
Mountain View, Calif.-based AliveCor on Thursday announced FDA clearance of KardiaBand in the U.S., allowing Apple Watch users to discreetly capture their EKG anytime, anywhere in order to quickly detect normal sinus heart rhythms and atrial fibrillation, the most common heart arrhythmia.
The first FDA-cleared medical device accessory for Apple Watch, KardiaBand can record an EKG in 30 seconds with just a touch of its integrated sensor. Results from the Kardia App are displayed on the face of Apple Watch.
-
EverlyWell jumps the ‘Shark’
“Shark Tank,” the hit ABC television series, is paying off for EverlyWell, an at-home health testing company. EverlyWell founder and CEO Julia Cheek accepted a $1 million line of credit in exchange for 5% equity on the show that aired Sunday, Nov. 26. It is the largest valuation deal for a solo female entrepreneur in “Shark Tank” history.