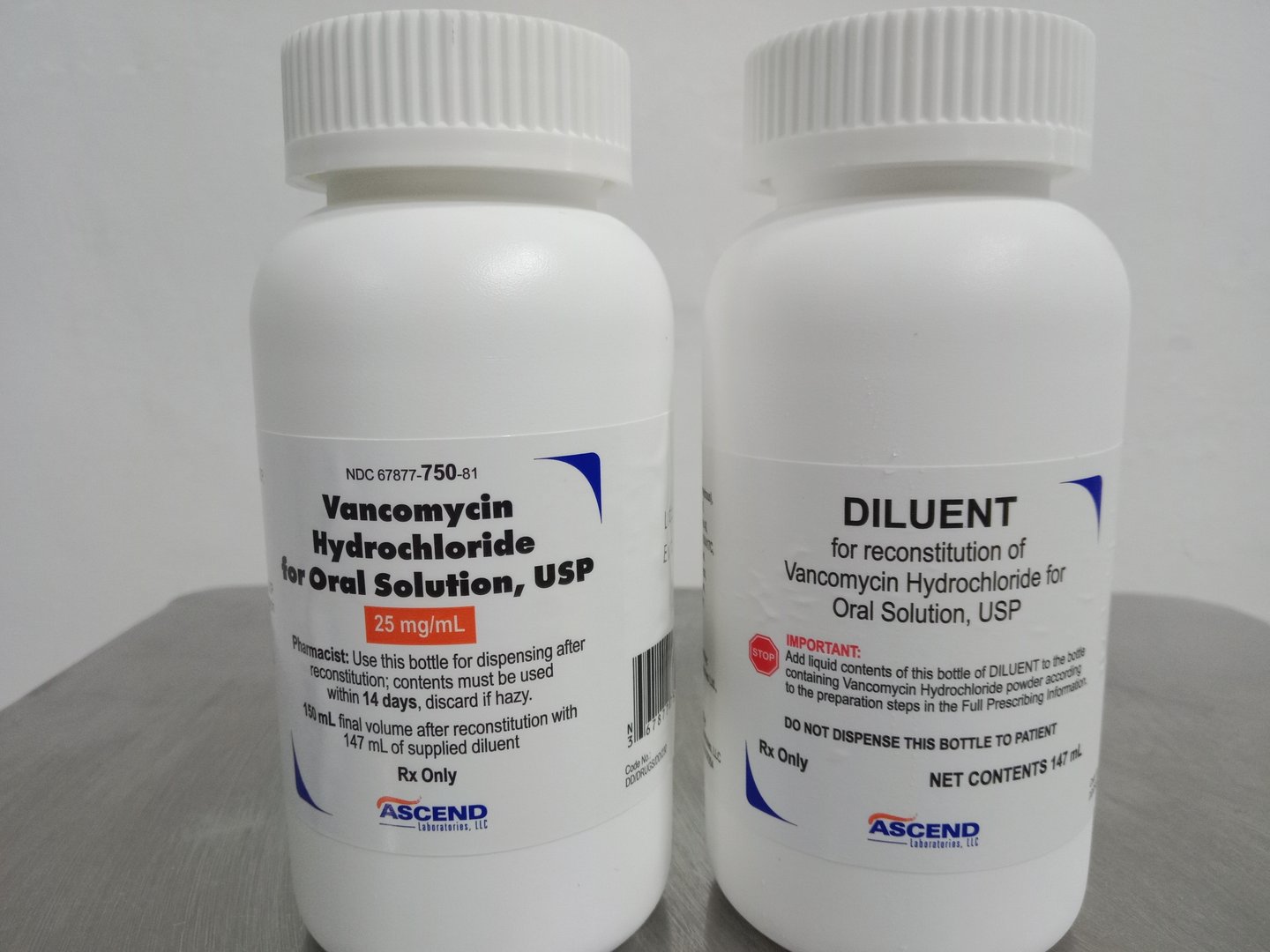
Ascend announces generic equivalent to Firvanq
Ascend Labs is pleased to announce its upcoming launch of Vancomycin for oral solution, the first vancomycin product with an approval comparable to Firvanq. Ascend has received FDA approval and anticipates a near term launch which will include an exclusivity period for being the first to file on this important drug. Ascend believes it will be the only ANDA generic available during this exclusivity period.
Ascend will launch the product in a 25mg strength in both a 150ml and 300ml size along with a 50mg also in 150ml and 300ml sizes. Your Ascend Account Executive will be contacting you with details on how to acquire this new to market generic product.
Advertisement - article continues below
Advertisement
For More Coverage: