12 exciting generic introductions that recently hit the market
Currently 90% of all prescriptions dispensed in the United States are for generic drugs, according to the annual report from the Office of Generic Drugs in the Center for Drug Evaluation and Research at the Food and Drug Administration. In 2021, the generic drug program approved hundreds of abbreviated new drug applications.
Following closely on the heels of FDA approvals, generic drug companies have launched a slew of generics, as well as biosimilars, in a broad range of therapeutic categories that include dermatology, oncology, antibiotics, ophthalmology and more. Here’s a look at the latest exciting introductions in the generic drug categories.
Accord Healthcare
Accord Healthcare has added carmustine to its line of chemotherapy drugs. The drug is formulated as a sterile lyophilized (freeze-dried) powder to be reconstituted for intravenous infusion.
Carmustine is the generic of Avet Pharmaceuticals’ BiCNU and is approved for use in the treatment of certain types of brain tumors and blood cancers.
Accord is offering carmustine in a 50-mg size, as well as the new, larger 300-mg strength, which was not previously available in the market and overcomes the potential need to use multiple vials of the smaller size, the company said.
“We are proud to be able to provide carmustine lyo. injection in a unique dosage size that is not currently available in the market,” said Kevin Congdon, Accord’s vice president of institutional sales. “Adding this chemotherapy drug to our portfolio illustrates Accord’s commitment in the U.S. market to support healthcare providers in prescribing accessible oncology medications.”
Carmustine lyo. injection is used alone or with other drugs to treat glioblastoma, astrocytoma, medulloblastoma and other types of malignant brain tumors; relapsed or refractory Hodgkin’s lymphoma; multiple myeloma, a blood cancer that affects the bone marrow; and relapsed or refractory non-Hodgkin lymphoma (the disease has recurred or has not gotten better with other treatment).
Alembic
Alembic recently launched two generic products: adapalene and benzoyl peroxide 0.3%/2.5% topical gel.
The medication, which is the generic of Galderma’s Epiduo Forte, is indicated for the topical treatment of acne vulgaris in adults and pediatric patients 12 years of age and older.
Alembic’s product comes in 45-g tubes.
Alembic also recently debuted diclofenac sodium 3% topical gel, which is the generic of Fougera’s Solaraze.
The medication is a nonsteroidal anti-inflammatory drug indicated for the topical treatment of actinic keratoses.
Diclofenac sodium topical gel is contraindicated in the setting of coronary artery bypass graft surgery, the company noted.
Alembic’s generic product is available in 100-g tubes.
Amneal
Amneal recently debuted several generic medications: Clindamycin phosphate gel, 1%, which is the generic of Clindagel, a dermatologic agent; atropine sulfate ophthalmic solution, 1%; famotidine for oral suspension, a gastrointestinal agent, which is the generic of Pepcid; micophenolate mofetil tablets, which is the generic of CellCept, an immunologic agent; and dexamethasone tablets, 2 mg, an anti-inflammatory agent.
In October, Amneal launched two biosimilars: Releuko (filgrastim-ayow), a biosimilar to Neupogen, and Alymsys (bevacizumab-maly), a biosimilar to Avastin. Both medications are oncology products typically administered in the acute care or clinic settings.
Releuko is a man-made form of granulocyte colony-stimulating factor indicated for neutropenia in patients undergoing chemotherapy.
Alymsys is a vascular endothelial growth factor inhibitor indicated for treatment of various cancers — mainly colorectal and non-squamous non-small cell lung cancer.
“The launch of Alymsys provides additional access and choice to affordable oncology therapeutics for providers and patients,” said Harsher Singh, senior vice president of Amneal Biosciences. “As a new entrant to the fast-growing $28 billion U.S. biosimilars market, we are leveraging our commercial expertise in the Buy and Bill injectable space and have built a dedicated biosimilar commercial team. We look to be an exceptionally customer centric organization and support stable, system-wide adoption of Alymsys and our biosimilars to come as a long-term player in this space.”
“Amneal has officially entered the U.S. biosimilar market. Beyond these initial launches, we are working to expand our biosimilars portfolio with additional molecules where we can be early to market and vertically integrate over time,” said Chirag and Chintu Patel, co-CEOs. “Biosimilars represent the next wave of affordable medicines and are closely aligned with our mission to provide high-quality, affordable medicines to as many patients as possible.”
ANI
ANI recently debuted prochlorperazine maleate tablets, the generic of Compazine.
The medication, which is used to treat the symptoms of psychosis and severe nausea or vomiting, was developed in technical collaboration with Biophore.
“We are pleased to bring prochlorperazine, another limited-competition product to the market and provide customers and patients access to high-quality generics,” said Nikhil Lalwani, president and CEO of ANI.
“In keeping with our mission of providing affordable, high-quality options for patients, we are pleased to partner with ANI on the launch of this limited-competition product,” said Jagadeesh Rangisetty, CEO of Biophore.
Prochlorperazine maleate tablets have a market value of roughly $30 million, according to IQVIA.
ANI also recently launched dexamethasone tablets in dosage strengths of 1.5 mg, 4 mg and 6 mg. The product is the generic of Decadron. Dexamethasone tablets have a market value of roughly $59.8 million, according to IQVIA.
Apotex
Apotex Corp is offering a new generic product. The company is presenting brimonidine tartrate/timolol maleate ophthalmic solution, which is a generic of Combigan.
Armas Pharmaceuticals
Armas Pharmaceuticals has unveiled oxacillin 2 g/vial.
Oxacillin for injection is a penicillin antibiotic used to treat staphylococcal infections. This is Armas’ second product in their antibiotic line and it follows the launch of piperacillin-tazobactam for injection in August.
The product has a market value of $6.5 million, according to IMS.
Aurobindo
Aurobindo recently launched dicloxacillin sodium capsules in dosage strengths of 250 mg and 500 mg.
The medication is the generic of Wyeth Ayerst’s Pathocil.
Dicloxacillin sodium capsules are indicated for the treatment of infections caused by penicillinase-producing staphylococci, which have demonstrated susceptibility to the drug.
Dicloxacillin sodium capsules have a market value of roughly $5.3 million for the 12 months ending July 2022, according to IQVIA.
Aurobindo said that the company is first to market with this product.
Camber
Camber has introduced several new generic products.
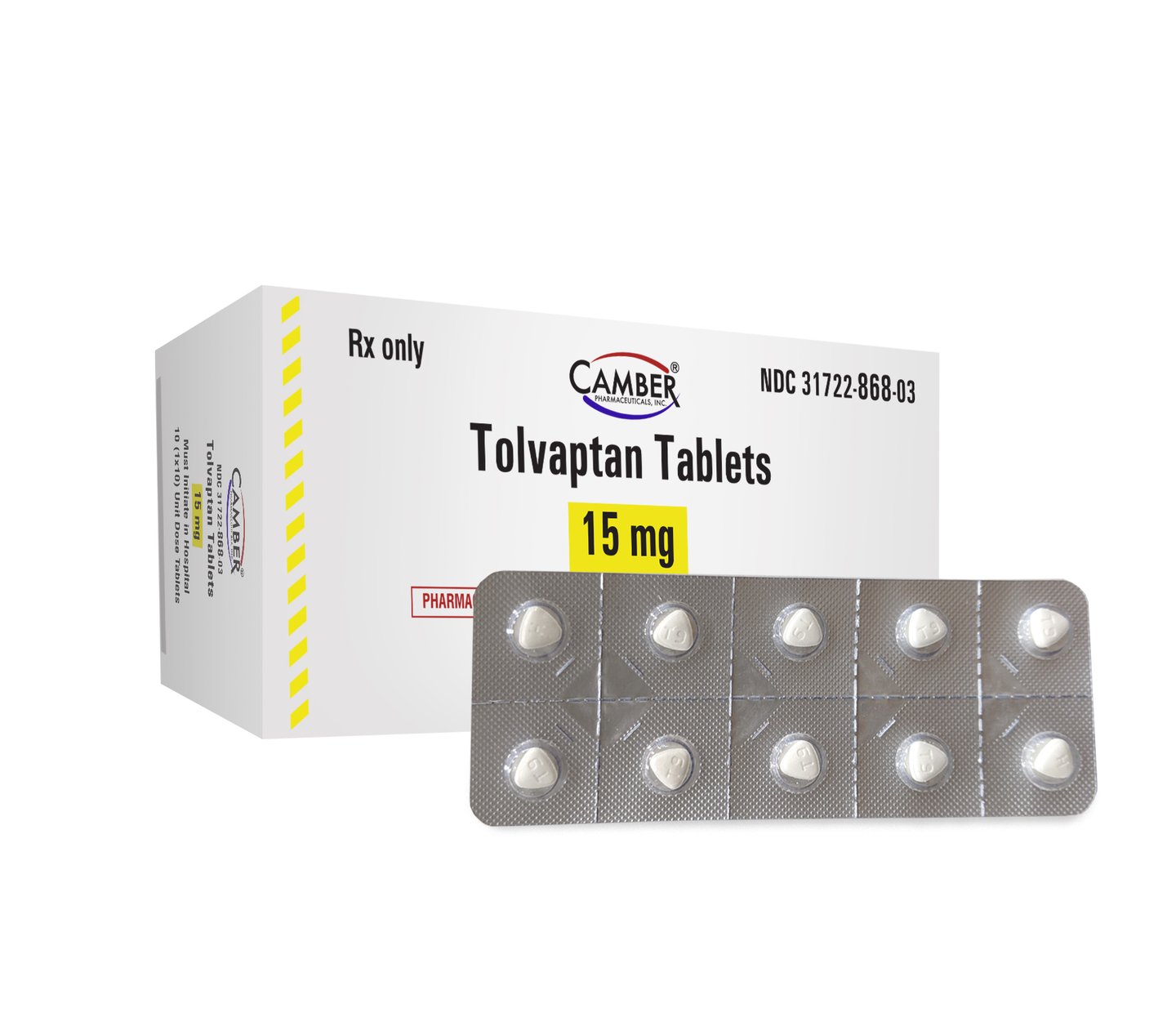
The company recently launched tolvaptan tablets, which are the generic of Otsuka’s Samsca.
The medication is used to treat hyponatremia (low levels of sodium in your blood) in people with heart failure and certain hormonal imbalances.
Camber’s tolvaptan tables are available in a dosage strength of 15 mg in a 10 (1x10) unit dose blister pack.
Camber also debuted solifenacin succinate tablets, which are the generic of Vesicare. The medication is used to treat symptoms of overactive bladder, such as frequent or urgent urination and incontinence (urine leakage).
Camber’s solifenacin succinate tablets are available in 5-mg and 10-mg strengths. Both strengths are available in 30- and 90-count bottles.
Lastly, Camber recently launched acyclovir oral suspension, a generic of Zovirax.
The medication is a synthetic nucleoside analogue active against herpes viruses.
Camber’s acyclovir oral suspension is available in 200-mg/5-ml strength in 473-ml bottles.
Celltrion
Celltrion USA plans to launch Vegzelma (bevacizumab-adcd), a biosimilar to Avastin (bevacizumab), in the first half of 2023.
The medication is used for the treatment of six types of cancer: metastatic colorectal cancer; recurrent or metastatic non-squamous non-small cell lung cancer; recurrent glioblastoma; metastatic renal cell carcinoma; persistent, recurrent or metastatic cervical cancer; and epithelial ovarian, fallopian tube or primary peritoneal cancer.
Coherus BioSciences
Coherus BioSciences is offering Cimerli (ranibizumab-eqrn), a biosimilar product interchangeable with Roche’s Lucentis (ranibizumab injection) for all approved indications.
An anti-VEGF therapy within a class of biologics that has been revolutionary in helping retinal patients maintain or gain vision, Cimerli was approved by the FDA in August 2022.
Cimerli is indicated for patients with neovascular (wet) age-related macular degeneration, macular edema following retinal vein occlusion, diabetic macular edema, diabetic retinopathy and myopic choroidal neovascularization.
Cimerli launched on Oct. 3, 2022, through U.S. specialty distributors at a list price of $1,360.00 and $816.00 per single-dose vial for the 0.5 mg and 0.3 mg dosages, respectively. This represents a 30% discount from the list price of the reference product, the company noted.
Additionally, through Cimerli Solutions, Coherus offers healthcare professionals comprehensive practice and patient support that includes patient assistance, electronic services and office support to ensure successful access and reimbursement.
“With the upcoming launch of Cimerli, retina specialists, patients and payers can expect the same efficacy and safety as Lucentis delivered with the comprehensive savings and patient support services that Coherus is known to deliver,” said Paul Reider, chief commercial officer of Coherus BioSciences
Dr. Reddy’s
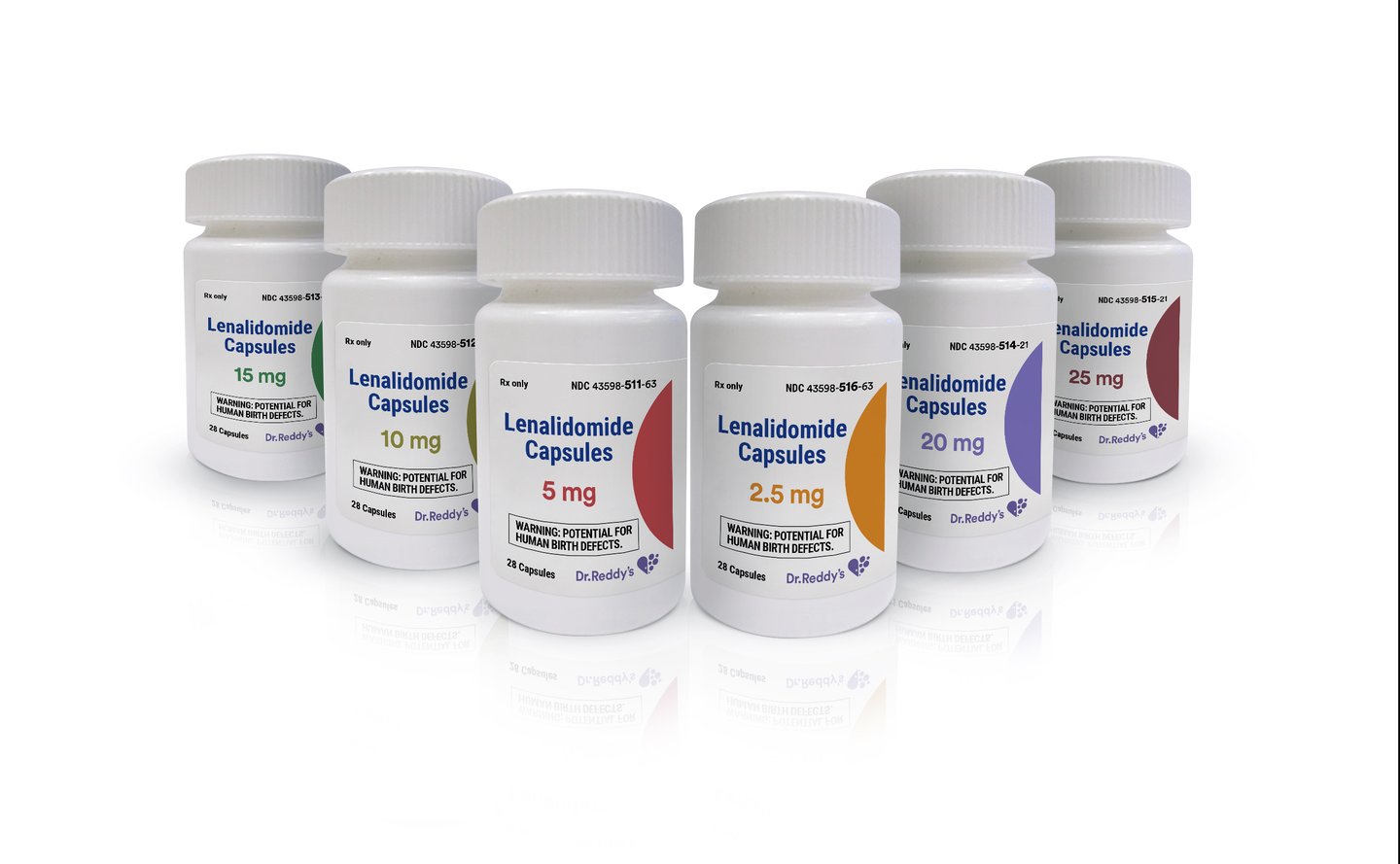
Dr. Reddy’s recently introduced lenalidomide capsules, a generic of Revlimid.
With this volume-limited launch, Dr. Reddy’s is eligible for first-to-market, 180 days of generic drug exclusivity for lenalidomide capsules in 2.5-mg and 20-mg strengths.
Revlimid is used to treat adults with multiple myeloma in combination with the medicine dexamethasone or as maintenance treatment after autologous hematopoietic stem cell transplantation, a type of stem cell transplant that uses your own stem cells.
As previously announced, Celgene agreed to provide Dr. Reddy’s with a license to sell volume-limited amounts of generic lenalidomide capsules in the U.S. in settlement of all outstanding claims of its litigation. The agreed-upon percentages remain confidential. As part of the settlement, Dr. Reddy’s also is licensed to sell generic lenalidomide capsules in the U.S. without volume limitation, beginning Jan. 31, 2026.
Dr. Reddy’s lenalidomide capsules are available in strengths of 2.5 mg, 5 mg and 10 mg, each in a bottle-count size of 28, as well as 15-mg, 20-mg and 25-mg strengths, each in a bottle-count size of 21.
Fresenius Kabi
Fresenius Kabi is offering gadoterate meglumine injection, a generic of Guerbet’s contrast agent Dotarem.
Contrast agents are used by radiologists to enhance the visibility of internal structures in imaging procedures such as MRI or CT scans. Contrast agents are a new category of healthcare products for Fresenius Kabi.
“Fresenius Kabi is pleased to expand our contrast agent portfolio and our support for the radiology community with the launch of gadoterate meglumine injection,” said John Ducker, president and CEO of Fresenius Kabi USA. “Contrast agents are vital to patient diagnosis, and the addition of Fresenius Kabi gadoterate meglumine will provide hospitals and clinics across the United States with more high-quality choices to support patient care.”
Gadoterate meglumine injection is a gadolinium-based contrast agent indicated for intravenous use with magnetic resonance imaging in brain (intracranial), spine and associated tissues in adult and pediatric patients (including term neonates) to detect and visualize areas with disruption of the blood brain barrier and/or abnormal vascularity.
The product is available in 5-, 10-, 15- and 20-ml single-dose vials.
Xiromed
Insud Pharmaceuticals’ Xiromed division is offering fulvestrant injection 250 mg/5 ml (50 mg/ml), a generic of Faslodex, which is used to treat advanced-stage, hormone receptor-positive, HER2-negative breast cancer.
Faslodex and its generics had a market value of $101.4 million for the 12 months ending July 2022, according to IQVIA.
“The launch of fulvestrant injection demonstrates Xiromed’s ability to leverage Insud Pharmaceutical’s breadth of manufacturing capabilities to bring high-quality, vertically integrated generic products to the U.S. market,” said Rob Spina, CEO of Xiromed.