-
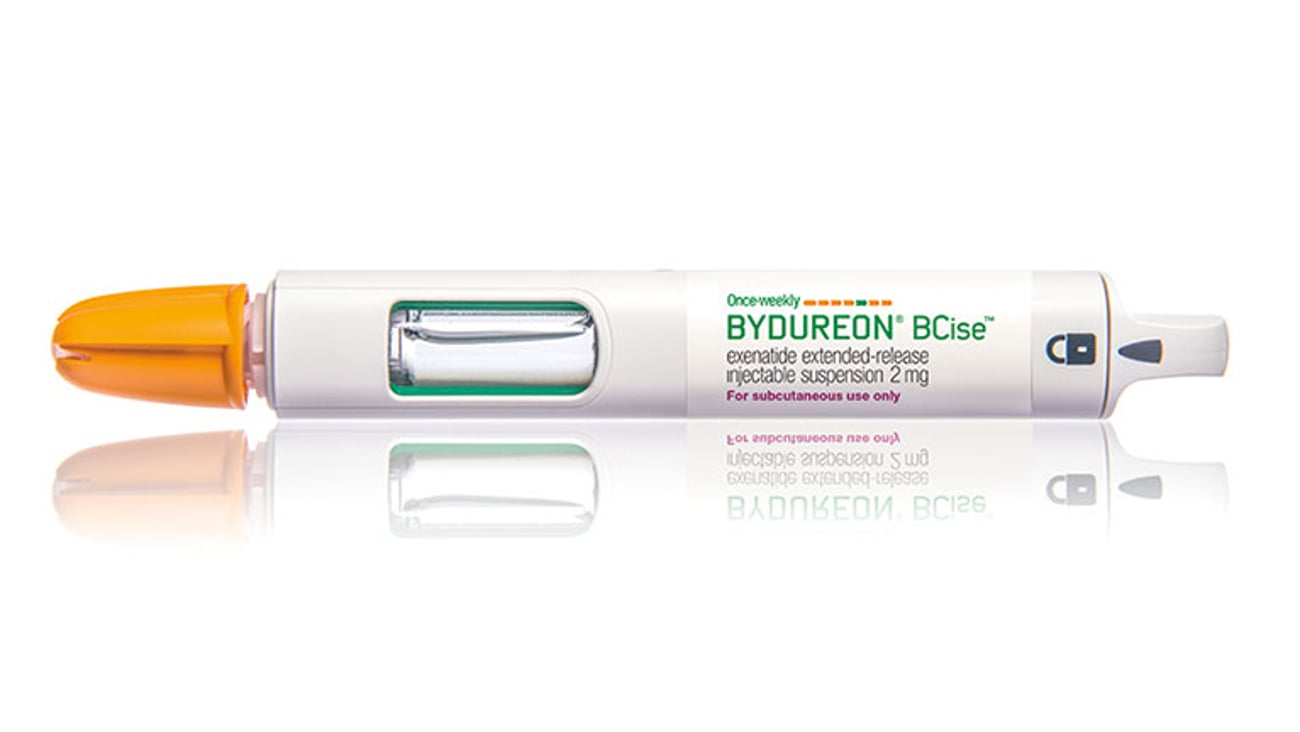
FDA approves new Bydureon formulation, auto-injector
SILVER SPRING, Md. — The Food and Drug Administration has approved an auto-injector and new formulation of a once-weekly treatment for Type 2 diabetes from AstraZeneca. Bydureon BCise offers a single-dose auto-injector for adults with Type 2 diabetes whose blood sugar is uncontrolled on one or more oral medicines.
-
GSK Vaccines set to bring Shingrix, the latest shingles vaccination innovation, to market
LONDON — GlaxoSmithKline on Friday announced that the Food and Drug Administration has approved Shingrix (zoster vaccine recombinant, adjuvanted) for the prevention of shingles (herpes zoster) in adults aged 50 years and older. Shingrix is a non-live, recombinant subunit vaccine given intramuscularly in two doses.