-
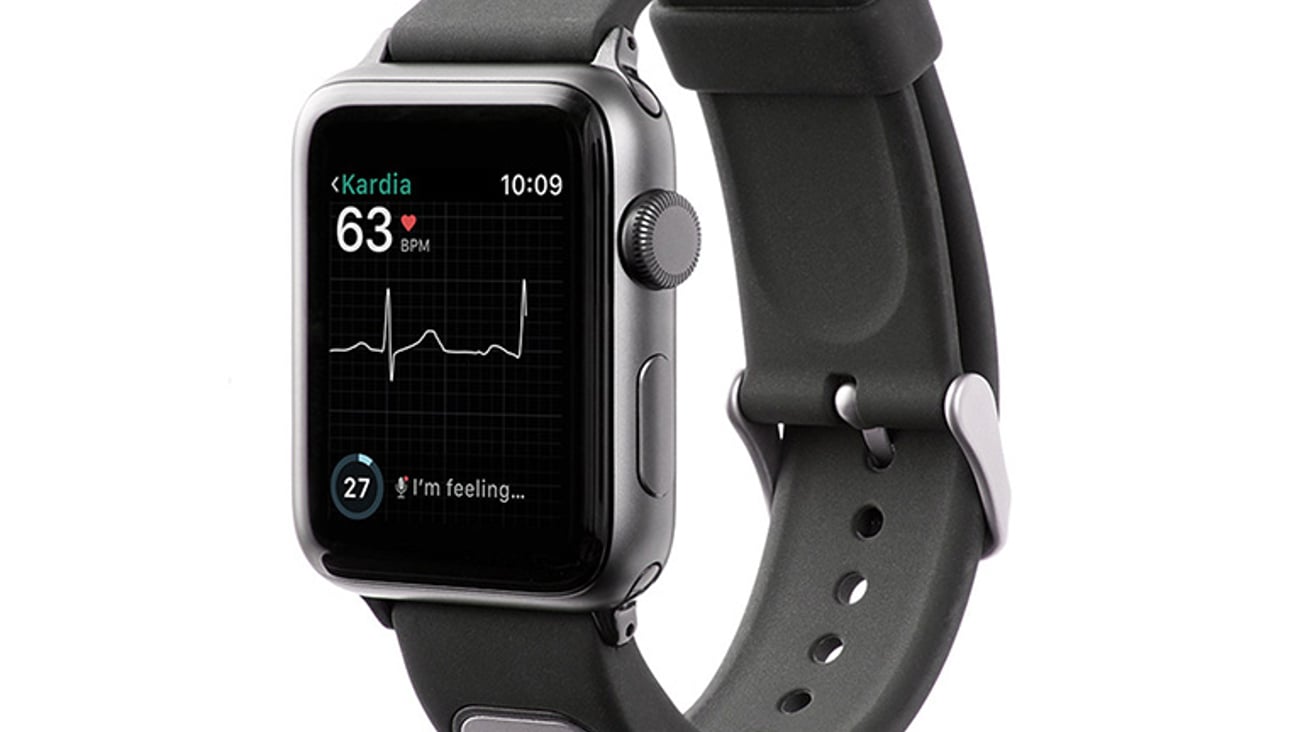
In a first for Apple, FDA clears EKG device for Apple Watch
Mountain View, Calif.-based AliveCor on Thursday announced FDA clearance of KardiaBand in the U.S., allowing Apple Watch users to discreetly capture their EKG anytime, anywhere in order to quickly detect normal sinus heart rhythms and atrial fibrillation, the most common heart arrhythmia.
The first FDA-cleared medical device accessory for Apple Watch, KardiaBand can record an EKG in 30 seconds with just a touch of its integrated sensor. Results from the Kardia App are displayed on the face of Apple Watch.
-
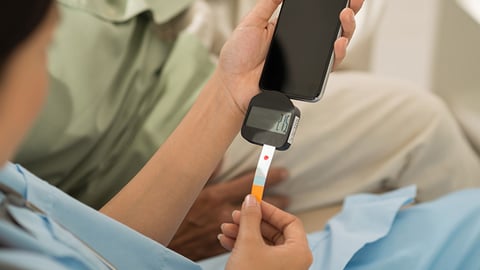
Trend toward self-management fuels growth of diabetes device sales
With the increasing trend of self-management and the rising awareness around the diabetes disease state within developing countries, blood glucose meters can be utilized to aid in adjustment of therapeutic regimen in response to blood glucose values and to help patients adjust their dietary intake, physical activity and insulin doses to improve glycaemic control on a regular basis.
This and several other factors are covered in the latest Research and Marekts report focused on diabetes care devices.