-
Study: Diabetes prevalence has doubled in past 25 years
BALTIMORE — Cases of diabetes and pre-diabetes in the United States have nearly doubled since 1988, suggests new research released Tuesday from the Johns Hopkins Bloomberg School of Public Health, with obesity apparently to blame for the surge. The researchers also found that the burden of the disease has not hit all groups equally, with alarming increases in diabetes in blacks, Hispanics and the elderly.
-
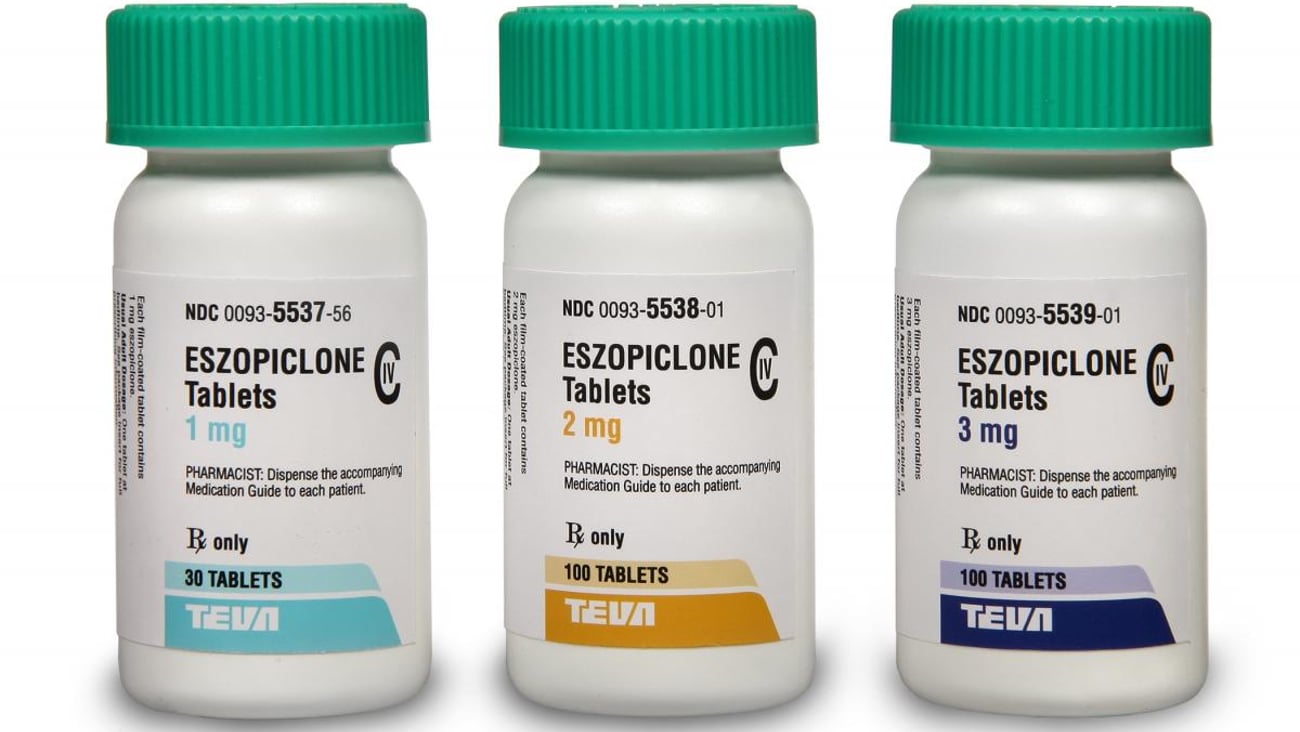
Teva launches generic Lunesta in the U.S.
JERUSALEM — Teva Pharmaceutical Industries announced the launch of a generic equivalent to Lunesta (eszopiclone tablets) in 1-,2- and 3-mg form in the United States. The drug is used to treat insomnia.
Lunesta tablets, which are marketed by Sunovion Pharmaceuticals, had annual sales of $852 million in the United States as of December 2013, according to IMS data.