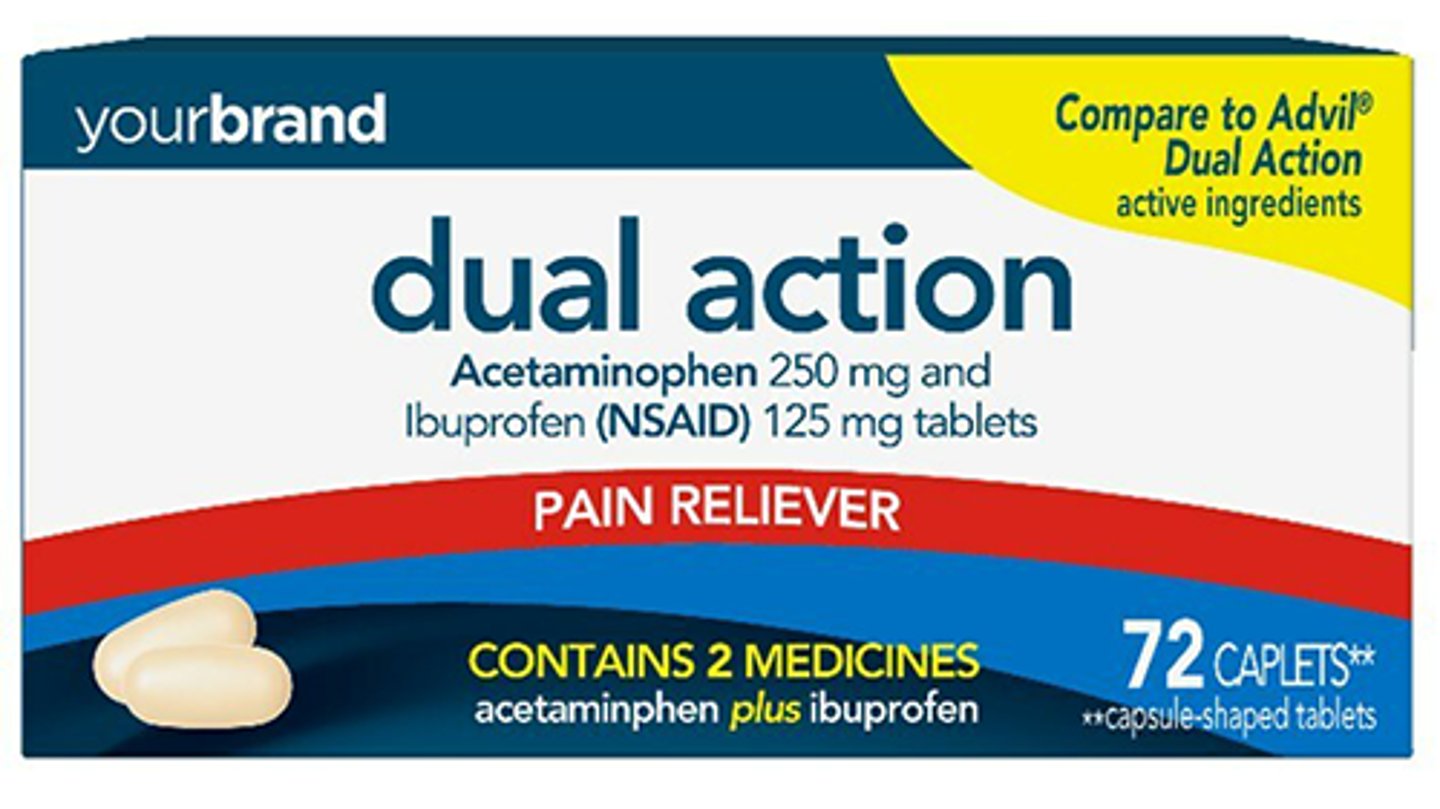
Perrigo receives FDA OK for store brand OTC Advil Dual Action tablets
Perrigo has received the Food and Drug Administration’s blessing for acetaminophen and ibuprofen tablets, 250 mg/125 mg, the store brand over-the-counter equivalent of Advil Dual Action tablets 250 mg/125 mg. The company anticipates launching the product by spring 2023.
"Following the successful approval and launch of Perrigo's first self-led branded prescription-to-OTC switch of Nasonex24HR, this prescription-to-OTC approval further exemplifies the strength of our regulatory and development capabilities, enabling our business to quickly follow national brand innovation and consumer preferences," said Jim Dillard, Perrigo executive vice president and president of consumer self-care Americas. "This approval was received on the first day possible following the expiration of the marketing exclusivity for Advil Dual Action tablets 250 mg/125 mg."
[Read more: Perrigo closes acquisition of Prevacid 24HR OTC rights]
Acetaminophen and ibuprofen tablets, 250 mg/125 mg, provide relief for multiple pain-related symptoms by combining two ingredients indicated for OTC pain relief.
Retail sales for the national brand equivalent product were roughly $69 million, for the last 12 months ending Jan. 29, 2023, according to IRI multi-outlet market data.