-
AP: Oklahoma legislator files bill requiring PBMs to get a state license from state pharmacy board
OKLAHOMA CITY — Legislation that would require pharmacy benefit managers to seek licensure from the Oklahoma State Board of Pharmacy if they were to distribute medicines to Oklahoma residents was introduced on Tuesday, according to an Associated Press report.
-
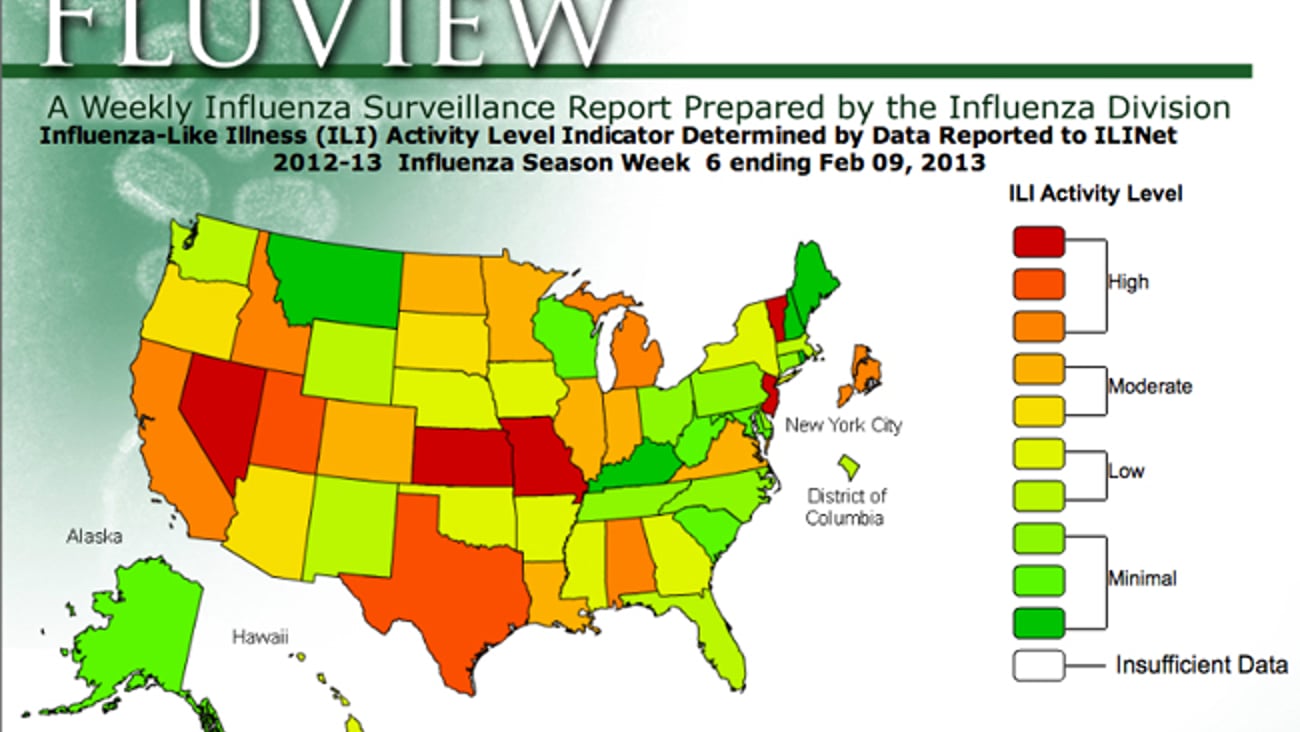
CDC: Flu activity drops to 3.2%, still above national baseline of 2.2%
ATLANTA — For the week ended Feb. 9, influenza activity decreased to 3.2% — still above the baseline of 2.2%, but an indicator this year's flu season is on its way out.
Eleven states and New York City were still experiencing high influenza-like illness activity, while 10 states reported moderate activity.