-
J&J exploring strategic options for LifeScan unit
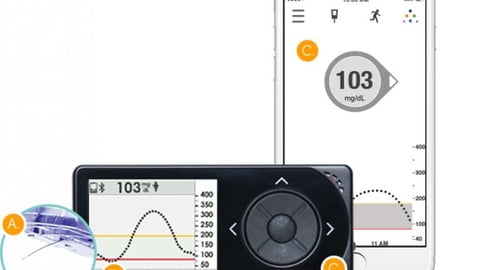
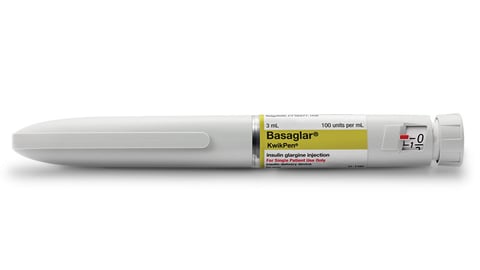
NEW BRUNSWICK, N.J. — Johnson and Johnson on Tuesday announced it will be evaluating "potential strategic options" for its LifeScan diabetes business, as well as other J&J diabetes banners including Animas Corp. and Calibra Medical.
The news was announced in conjunction with the company's fourth quarter earnings call, and is part of the company's ongoing portfolio management.