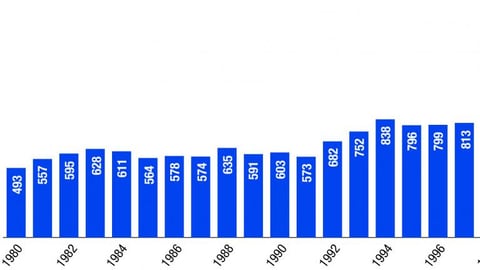
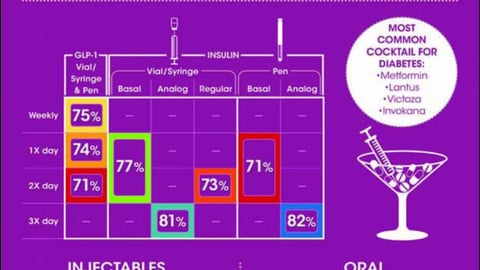
-
FDA approves Novo Nordisk’s Xultophy 100/3.6 for Type 2 diabetes
SILVER SPRING, Md. — Novo Nordisk announced Monday that the Food and Drug Administration had approved its Xultophy 100/3.6 (insulin degludec 100 units/ML and liraglutide 3.6 mg/mL). The drug is indicated to improve glycemic control in adults with Type 2 diabetes inadequately controlled on less than 50 units of basal insulin daily or less than or equal to 1.8 mg of liraglutide daily.Sanofi’s Soliqua 100/33 for Type 2 diabetes gets FDA nod
SILVER SPRING, Md. — The Food and Drug Administration has approved Sanofi’s Soliqua 100/33 (insulin glargine 100 units/mL and lixisenatide 33mcg/mL), the company announced Monday. The drug is indicated to treat adults with Type 2 diabetes that’s inadequately controlled on basal insulin or lixisenatide.