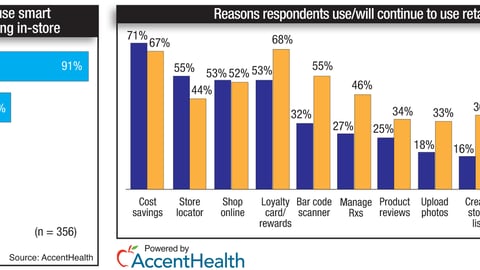
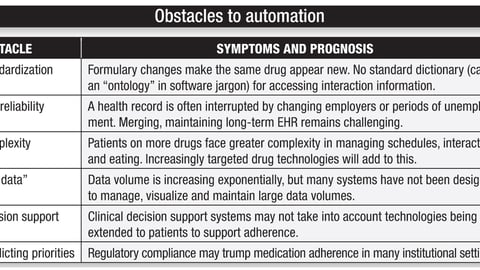
-
Parkland Health Mart earns Pharmacy of the Year Award
Quantity and quality: Those are the two metrics by which any pharmacy operation is measured — quantity of prescriptions dispensed and quality of services provided. Quantity keeps you in business; quality keeps your patients coming back.
It’s a constant challenge for a small operator to score high on both metrics, but that’s exactly what McKesson’s 2013 Pharmacy of the Year Award recipient Parkland Health Mart Pharmacy, of Desloge, Mo., has done.
-
Skin care benefits brighten sales
 Category saturation and competition from private label may be inhibiting sales growth within the sun care segment, but infusing additional skin care benefits, like antioxidants, and targeting such demographic groups as men and multicultural consumers could help brighten sales.
Category saturation and competition from private label may be inhibiting sales growth within the sun care segment, but infusing additional skin care benefits, like antioxidants, and targeting such demographic groups as men and multicultural consumers could help brighten sales.(For the full category review, including sales data, click here.)