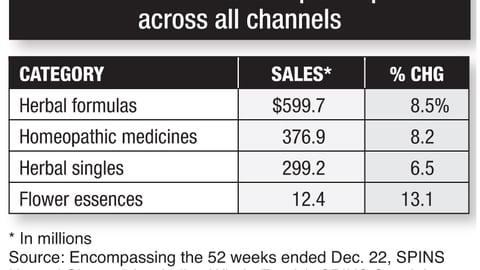
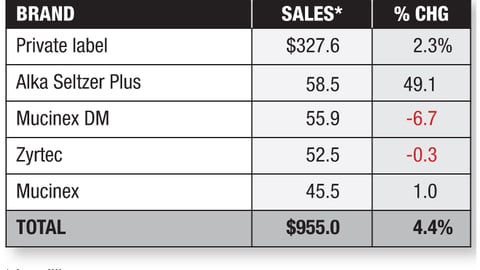
-
Office of the National Coordinator recognizes Michigan e-prescribing effort
LANSING, Mich. — The federal government has recognized the state of Michigan as a national leader in electronic prescribing, a group of health organizations in the state said.
The Michigan Health Information Network said that the Office of the National Coordinator for health information technology had commended it, recognizing that 94% of pharmacies in the state are equipped for e-prescribing.