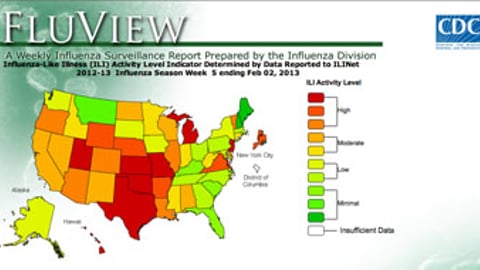
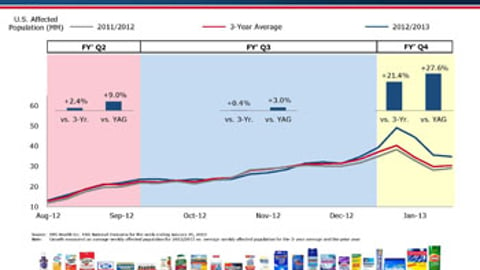
-
FDA approves generic diabetes drug
SILVER SPRING, Md. - The Food and Drug Administration has approved a generic diabetes drug made by Macleods Pharma, according to agency records.
The FDA approved Macleods' pioglitazone hydrochloride tablets in the 15 mg, 30 mg and 45 mg strengths.
The drug is a generic version of Takeda's Actos, branded and generic versions of which had sales of about $2.7 billion during the 12-month period that ended in August 2012, according to IMS Health.
-
Sanofi: Rolaids will be relieving heartburn with its return to the market by the end of the year
PARIS — During a quarterly conference call Thursday, Sanofi president of global operations Hanspeter Spek noted that Sanofi's consumer arm Chattem will be relaunching the heartburn remedy Rolaids.
"We plan to relaunch the product, which has been absent of the American markets for production problems on the side of J&J," he told analysts. "We intend to launch the product by the end of 2013, which will further accelerate the performance of Chattem in the United States."