-
Nanowear gains FDA approval for first-of-its-kind wearable: undergarment that tracks cardiac health
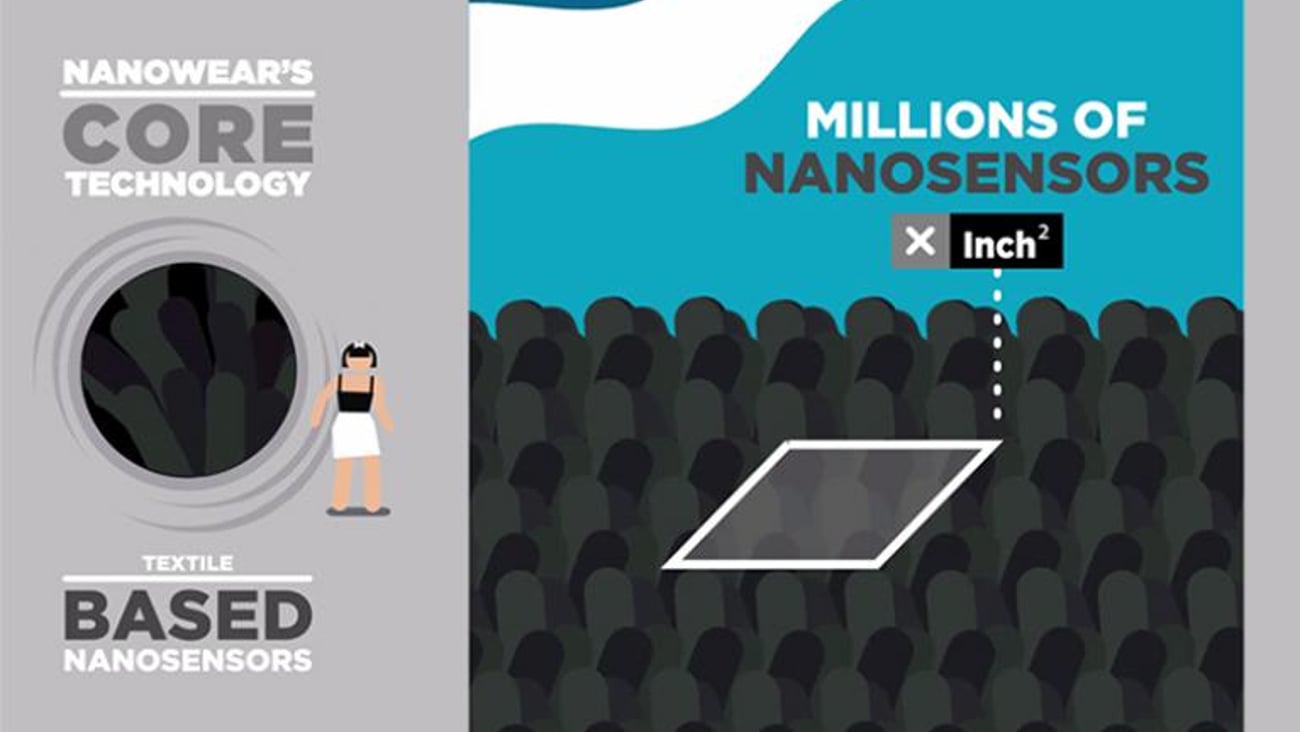
NEW YORK -- Nanowear, an early stage developer of cloth-based diagnostic monitoring nanosensor technology, on Thursday announced that it has received FDA Class II 510(k) clearance for its first product, SimplECG, a remote cardiac monitoring undergarment.
SimplECG collects continuous multi-channel ECG, heart rate and respiratory rate data from the garment and transfers it to a web-based portal for review by a physician, by way of a mobile application. The initial version of the product is iPhone-based.